Is Baking Soda an acid or base?
Baking soda is an interesting chemical. It has the characteristic texture of a white solid fine powder that has some properties that make it seem like it should be an acid, but others that make it seem like a base.
So which one is it? Is baking soda acid or a base?
Baking soda is a base!
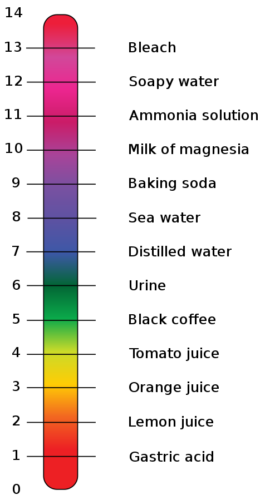
Anything with a ph level of over 7 is a base and baking soda’s ph level is around 9.
Baking soda is a chemical compound with the formula NaHCO. This means that it has one atom of sodium, which is a metal and an alkali earth element (group IA), attached to one atom of hydrogen carbonate, or HCO-.
Since baking soda has an excess positive charge, it is considered to be a base. This means that it will react with acids to neutralize them. For example, if you have a recipe that calls for baking soda and vinegar, the two chemicals will interact to produce carbon dioxide gas (CO) and water vapor (H).
What is sodium carbonate?
Sodium carbonate is known as washing soda. It’s a basic salt and is the building block of baking soda.
Baking Soda and Carbon Dioxide equals fluffy bread
Baking soda has a variety of uses in the kitchen. It’s commonly used in baking to help leaven different types of dough by creating carbon dioxide when combined with an acid, such as sour cream or buttermilk.
The sodium bicarbonate reacts with these acids and releases bubbles that are trapped within the batter causing it to rise.
Basically, the alkaline properties of baking soda combine with an acidic solution and make your bread nice and fluffy!
That’s right, it’s not just heat or the cooking process that causes your bread to rise, but science!
In addition to baking, you can also use baking soda as a cleaning agent for many different surfaces in your home.
Why is Baking Soda good for cleaning?
This chemical reaction caused by sodium bicarbonate can also be used to clean many surfaces around your home but is especially great for those tough to clean surfaces like bathrooms and ovens.
Sprinkle some baking soda on the area and spray vinegar on it. Let it sit until it’s dissolved. The acid in the vinegar will react with the baking soda to produce CO and H, which loosen the dirt and crusted food on oven walls and quickly destroy mildew and grime on bathroom tile. Then you clean it up by spraying water on it. Dirt will just wash away.
The most famous use of baking soda for cleaning has to do with brushing your teeth! Brushing your teeth with baking soda can keep tooth decay at bay and keep your mouth healthy. And because its alkaline nature neutralizes acids, it can help cover bad breath.
What is the PH of Baking Soda?
Baking soda is a base with a pH level of about 9. Remember back to science class. Lower numbers are acidic, 7 is neutral, and anything above 7 is alkaline.
Can Baking Soda alkalize the body?
There is some debate over whether it can make your body more alkaline, but there is some evidence to suggest that it might be able to.
Some people believe that consuming baking soda can help to neutralize the acid in your stomach, helping you with any stomach problems you might have and improving your indigestion.
What’s the difference between Baking Powder and Baking Soda?
Baking powder is a leavening agent that contains baking soda, but it also has a weak acid in addition to sodium bicarbonate. Essentially, this creates gas when mixed with water and heated which expands during cooking to create air bubbles within your batter resulting in baked goods that are fluffier than they would have been otherwise.
Baking soda is a leavening agent that contains sodium bicarbonate and nothing else, so it doesn’t add any additional flavors to your baked goods but you must use an acid in the recipe or acidic ingredients like lemon juice if you want something more than a plain cake or bread.
When substituting one for the other, it’s important to remember that the two have different volumetric measures – you’ll need less baking powder than baking soda.
Why is it important to know if baking soda is an acid or base?
Baking soda is a bicarbonate of soda, which neutralizes acidity. This means that if you are baking something and it calls for baking soda, but you accidentally use baking powder instead (which already contains soda), your recipe will be very bitter.
If you are using an acidic ingredient in a recipe, such as lemon juice or chocolate, you may need to balance it out by adding more baking soda.
What is Baking Soda made of?
Baking soda is made of sodium bicarbonate, which is a combination of a strong alkali and a weak acid, so it acts as a weak alkali. The strong alkali is sodium hydroxide and the weak acid is carbonic acid (also known as soda water). Putting these two together makes the solution known as sodium carbonate, also known as washing soda.
Then, when you take more sodium carbonate and add even more carbonic acid (or bicarbonate ions), you get sodium bicarbonate.
Final Thoughts
So, is baking soda acid or base? It’s a base because it has a ph level of about 9. But no matter your background in science, it’s cool to see how chemistry can be used to cook delicious food.
Although the Easy Kitchen Appliances team is more familiar with bread makers than beakers, we hope that this has been an informative article.
Related Posts
-
Top 7 Best Baking Powder Substitutes
Looking for an easy substitute for baking powder? There are times when we find ourselves…
-
Bread Machine Cycle Times
Everything You Need to Know About Bread Machine Cycles and Cycle Times Making bread can…
-
How to Use a Convection Oven for Baking Cakes
One of the greatest things about convection ovens is their versatility and the ability to…






